Clinical Trial Management System
Your central hub for monitoring and managing clinical trial progress with ease and efficiency. Our intuitive dashboard offers real-time insights into your trials, helping you stay on top of all key metrics and activities.
Why Choose Our CTMS?
Simplify complex processes, optimize performance, and ensure successful outcomes—all in one place.
Efficiency & Transparency
Streamline your clinical trial processes and improve workflow with real-time visibility into every aspect of your trials.
Regulatory Compliance
Built-in compliance features and other global standards along with 21CFR Part 11. Audit trails and data encryption channel to ensure privacy.
Data Integrity
Safeguard your trial data with advanced security measures and automated validation tools, reducing the risk of errors and enhancing compliance.
Scalability
Whether you are managing one trial or hundreds, our CTMS grows with your needs. Easily manage multiple trials, phases, and sites with a single platform.
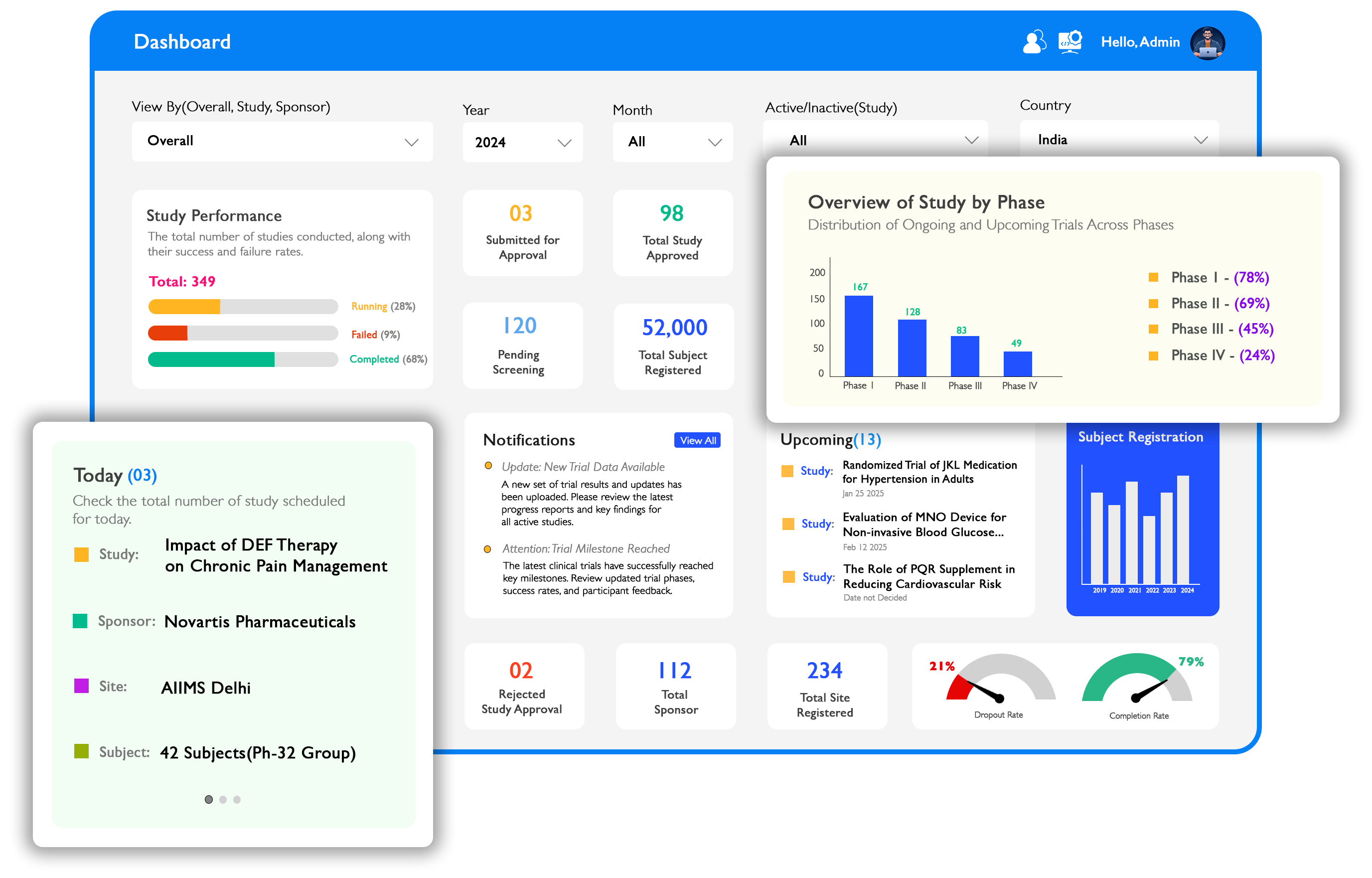
Powerful Dashboard for Complete Control
Our intuitive dashboard gives you a comprehensive overview of all your clinical trials in one place. Monitor key metrics, track progress, and identify potential issues before they become problems.
Real-time monitoring of trial progress
Customizable views for different stakeholders
Interactive data visualization and reporting
Automated alerts for critical events
Clinical Trial Dashboard
Trial Progress Overview
Recent Notifications
Trial ABC-123 requires attention
2 hours ago
Phase II completed for Trial XYZ-789
Yesterday
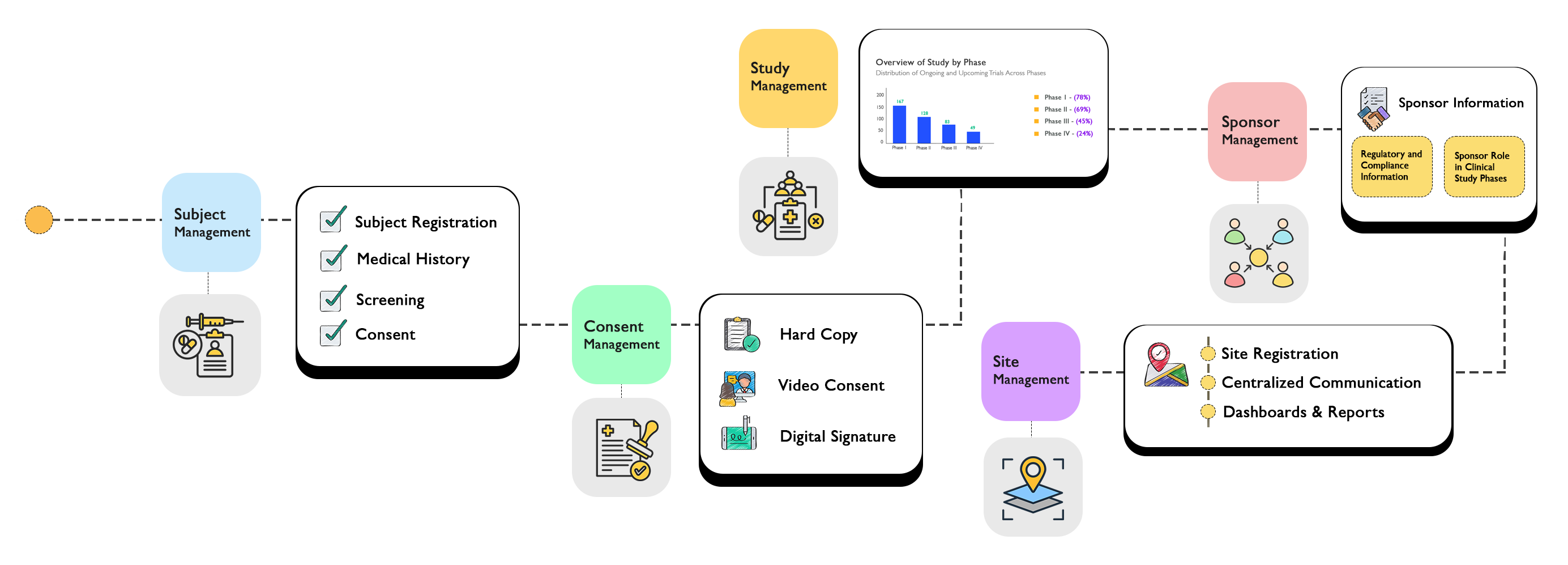
Comprehensive System Architecture
Our CTMS provides a complete ecosystem for managing every aspect of your clinical trials.
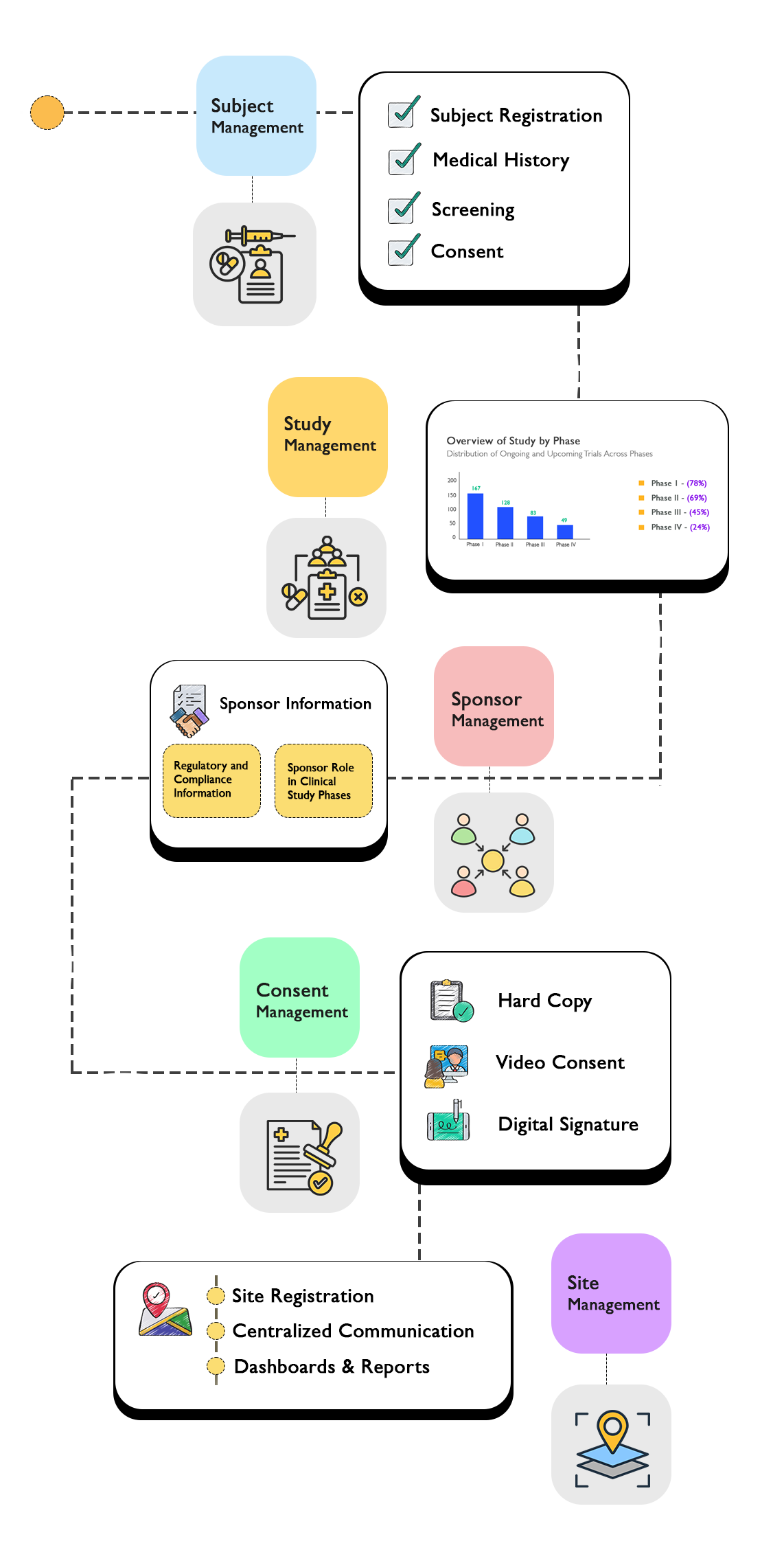
Comprehensive Feature Set
Everything you need to manage your clinical trials efficiently and effectively.
Document Management
Store and organize all trial-related documents in one secure, centralized location. Easily access contracts, ethics approvals, consent forms, and regulatory submissions.
- Centralized document repository
- Version control and audit trails
- Electronic signatures and approvals
- Automated document workflows
- Advanced search and filtering
Document Repository
Study Protocol v2.1
Updated 2 days ago
Informed Consent Form
Updated 1 week ago
Case Report Form
Updated 3 days ago
Regulatory Submission
Updated 5 days ago
Get Started with Your Free Demo
See how our Clinical Trial Management System can transform your trial operations. Schedule a personalized demo today and discover the difference.